Before 2012, waiting for a generic drug to hit the market could take years. Backlogs piled up. Applications sat untouched. Patients waited longer for affordable medicines. Then came the Generic Drug User Fee Amendments-or GDUFA-a law that changed everything. It didn’t just tweak the system. It rebuilt it from the ground up, using money from drugmakers to fund faster, more predictable reviews by the FDA.
What GDUFA Actually Does
GDUFA isn’t a vague policy. It’s a binding agreement between the FDA and generic drug companies. In exchange for paying fees, manufacturers get clear timelines, dedicated staff, and faster decisions. The FDA, in turn, gets the money it needs to hire more reviewers, upgrade technology, and inspect factories-especially overseas ones that make most of America’s generic drugs. The law was signed in 2012 as part of the Food and Drug Administration Safety and Innovation Act. It was the first time the FDA could legally charge generic drug makers for review services. Before that, the agency relied entirely on Congress for funding, which meant delays, budget cuts, and unpredictable timelines. GDUFA fixed that.How the Fees Work
Not all fees are the same. The system is built around three main types:- Facility fees: Paid yearly by every plant that makes generic drugs. Domestic facilities pay less than foreign ones. In 2024, a U.S. finished drug facility paid $208,600. A foreign one paid $223,600. The $15,000 difference covers the extra cost of inspecting overseas sites.
- Application fees: A one-time charge when you submit an ANDA (Abbreviated New Drug Application). This is the formal request to sell a generic version of a brand-name drug.
- Drug Master File (DMF) fees: Paid when a supplier’s chemical or manufacturing details are first referenced in an application.
Why Foreign Facilities Pay More
About 70% of the active ingredients in U.S. generic drugs come from outside the country-mostly India and China. The FDA inspects these foreign plants, too. But it’s more expensive. Travel, translators, logistics, and longer inspection times add up. So GDUFA charges foreign facilities $15,000 more per year than U.S. ones. That’s controversial. Some companies say the gap is outdated. They argue that modern inspections are more efficient, and the extra fee doesn’t reflect real costs. Others, including the FDA, say it’s still necessary. The agency has inspected over 2,000 foreign facilities since 2012-many more than before GDUFA.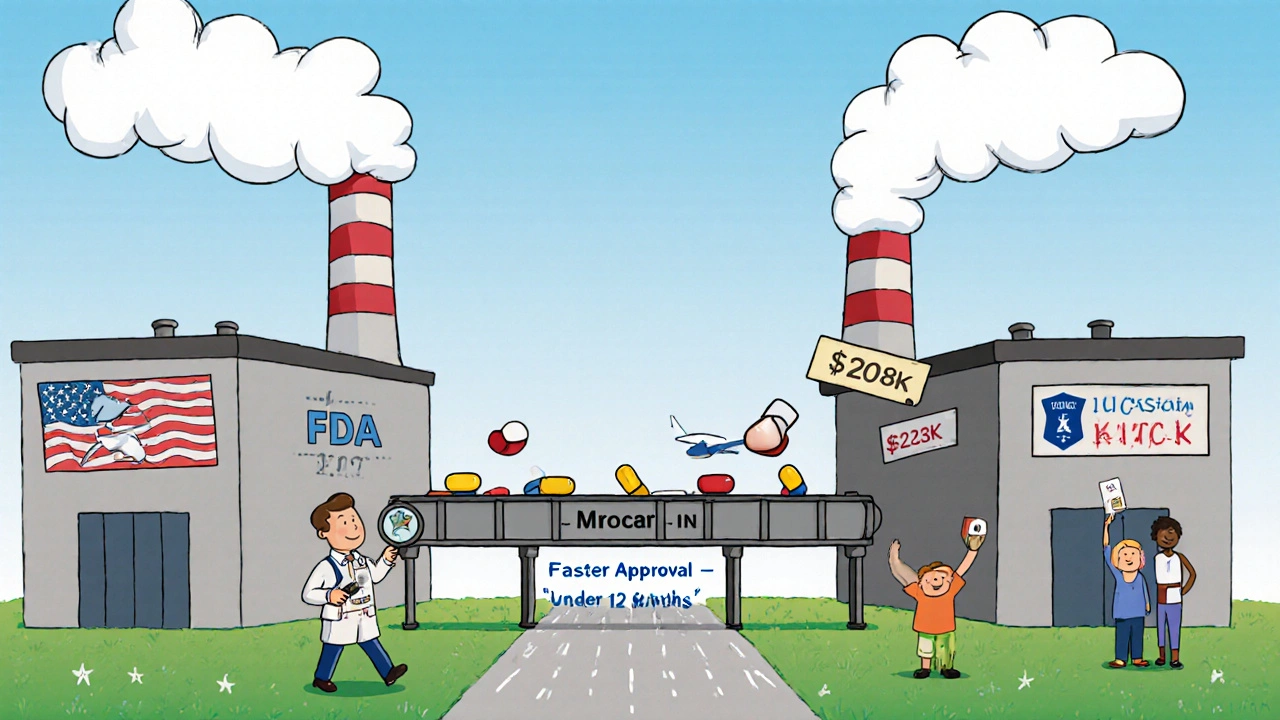
How GDUFA Changed the Game
Before GDUFA, the average review time for a generic drug application was over 30 months. In 2024? It’s under 12 months. That’s not a coincidence. The law forced the FDA to set clear goals:- Review 90% of applications within 10 months
- Inspect 75% of domestic facilities every 2 years
- Inspect 50% of foreign facilities every 3 years
Who Gets Hurt? The Small Players
Big companies with dozens of products spread the cost of GDUFA fees across many applications. But small startups? One facility fee can cost more than their entire annual budget. That’s why GDUFA II (2018) added discounts for small businesses. If you have fewer than 500 employees and fewer than 10 ANDAs, you get a 90% reduction on your facility fee. That helped. But it didn’t fix everything. Many smaller manufacturers still struggle. One company in Ohio told the FDA they had to delay launching three generics because they couldn’t afford the fees. The FDA acknowledges this. That’s why GDUFA III (2023) introduced new tools like the Pre-ANDA Program. It lets small firms meet with FDA scientists before submitting their full application-saving time and money.What’s New in GDUFA III (2023-2027)
GDUFA III didn’t just tweak fees. It added whole new programs:- Pre-ANDA Program: Early meetings with FDA experts to avoid costly mistakes.
- ANDA Assessment Program: Real-time feedback during the review process.
- Controlled Correspondence: A faster way to ask questions without formal letters.
- Drug Master File Assessments: Faster review of ingredient suppliers.
Industry Reactions
Big manufacturers love GDUFA. Predictability means better planning. Lower risk. Higher profits. Smaller companies are mixed. They appreciate the Pre-ANDA Program and fee discounts. But they still say the system favors scale. One consultant in Texas told us, “If you’re not a top-10 generic maker, you’re still fighting an uphill battle.” The FDA says they’re listening. They’ve held over 20 public meetings since 2022 to hear from small businesses. But change is slow. Fees are set by law. Only Congress can adjust them.The Bigger Picture
GDUFA isn’t just about speed. It’s about access. The U.S. spends $150 billion a year on generic drugs. Without them, Medicare and private insurers would pay hundreds of billions more. The law also changed how the industry works. Since 2012, the top 10 generic drugmakers now control over half the market. Why? Because only big players can handle the compliance costs. New entrants find it harder to break in. Some experts worry this concentration could hurt competition. Others say it’s the price of efficiency. The FDA’s data shows more drugs approved, faster. That’s good for patients.What’s Next? GDUFA IV
GDUFA III expires in September 2027. Negotiations for the next version-GDUFA IV-are already starting. Potential changes? Lower fees for small businesses. Digital-only submissions. More support for complex generics like inhalers and injectables. The FDA says it wants to make the system even more predictable. But the big question remains: Will Congress keep funding this model? So far, yes. GDUFA has bipartisan support. Both parties agree: Americans need affordable drugs. And GDUFA delivers them.What is GDUFA and why does it matter?
GDUFA stands for the Generic Drug User Fee Amendments. It’s a law that lets the FDA collect fees from generic drug manufacturers to fund faster reviews of generic drug applications. Before GDUFA, approvals took years. Now, most are approved in under a year. It matters because it gets safe, affordable medicines to patients faster-and saves the healthcare system billions.
Who pays GDUFA fees?
Generic drug manufacturers pay GDUFA fees. This includes companies that make finished drug products (like pills or injections) and those that produce the active ingredients. Both U.S. and foreign facilities pay, but foreign ones pay more to cover the higher cost of international inspections.
How much do GDUFA fees cost in 2025?
In fiscal year 2025, the facility fee for a domestic finished drug facility is $208,600. For a foreign facility, it’s $223,600. Application fees for an ANDA are $177,500. These rates are set by the FDA and published annually in the Federal Register.
Does GDUFA help small generic drug companies?
Yes, but not perfectly. GDUFA II introduced a 90% fee discount for small businesses with fewer than 500 employees and fewer than 10 drug applications. GDUFA III added the Pre-ANDA Program to help them avoid costly mistakes early. Still, many small firms say the costs remain a barrier compared to large competitors.
How has GDUFA improved FDA inspections?
Before GDUFA, the FDA inspected only about 100 foreign generic drug facilities per year. Now, thanks to user fee funding, they inspect over 400 annually. Domestic facilities are inspected every two years, foreign ones every three. This has dramatically improved drug safety by catching quality issues before products reach patients.
When does GDUFA III expire?
GDUFA III expires on September 30, 2027. After that, Congress must pass new legislation to renew the program. Negotiations for GDUFA IV are expected to begin in 2025, with input from industry, patient groups, and the FDA.


Kaleigh Scroger
November 27, 2025 AT 22:48GDUFA was a game changer no doubt but people forget how bad it was before 2012 I mean we had applications sitting for years while patients went without life saving meds because Congress couldn't figure out how to fund the FDA properly
Now we get 1500 approvals a year and inspections overseas are actually happening instead of just promises
Yeah the fees are steep but if you're a big pharma company you're making billions off generics anyway so cough up the cash
And don't even get me started on how foreign facilities pay more it's not discrimination it's logistics
Inspecting a plant in Mumbai takes way more than just a plane ticket you need translators local guides customs coordination and time to recover from jet lag
The FDA doesn't make these rules up they're based on real cost data
Also the Pre-ANDA program is a lifesaver for small firms I know a guy who saved over $200k by using it
Before that he wasted a year and half on a rejected application because he didn't know the FDA expected a certain impurity profile
Now they sit down and talk before you even file
It's not perfect but it's lightyears ahead of what we had
Elizabeth Choi
November 29, 2025 AT 22:18Let's be real GDUFA didn't improve anything it just shifted the burden from taxpayers to generic manufacturers who now have to pay to get their own products reviewed
It's regulatory capture disguised as efficiency
The FDA became a fee-collecting bureaucracy instead of a public health agency
And now the same 10 companies dominate the market because only they can afford the compliance overhead
That's not innovation that's consolidation
Also why are we letting a government agency charge private companies for services that should be funded by the public
It sets a dangerous precedent
Emma louise
December 1, 2025 AT 21:00Oh wow so now we're paying Indian and Chinese factories more to make our cheap pills because we're scared of their quality
That's rich
Meanwhile our own drug supply chain is collapsing but we're fine with paying extra to keep foreign plants in business
What a joke
Next they'll charge us for breathing clean air
And don't tell me about inspections I've seen the reports
They send some guy with a clipboard for two days and then approve everything
It's theater
And you call this transparency
Pathetic
Alex Hess
December 2, 2025 AT 22:29Ugh another boring government report about fees and timelines
Can we just move on
Everyone knows generics are cheaper
Why does this even matter
It's not like I'm going to read the Federal Register before I fill my prescription
Just let me get my pills
And stop making me feel guilty for wanting affordable medicine
Lauren Zableckis
December 3, 2025 AT 17:16I appreciate the transparency GDUFA III brought to the process
Monthly reports on review times and inspections? That’s huge
Before we had no idea what was happening behind the scenes
Now you can track your application like a package
It’s not perfect but it’s a step toward accountability
And the Pre-ANDA program is actually useful
I’ve seen small companies avoid costly rejections because they got early feedback
It’s not about big vs small
It’s about reducing wasted time and money for everyone
Even if you’re a big player you don’t want to wait two years for approval
Efficiency benefits the whole system
reshmi mahi
December 4, 2025 AT 16:47lol USA charging us more to make your pills 🤡
India makes 40% of your generics and you act like we're stealing from you
Meanwhile your FDA inspectors show up with 3 translators and a 500 page checklist
Why don't you just build your own factories here
Oh right because labor is expensive and you'd have to pay workers more than $3 a day
So you tax us instead
Classic
Also 223k for a facility fee? Bro I could start a whole pharmacy in Bangalore for that
laura lauraa
December 5, 2025 AT 13:39It is, indeed, a profoundly troubling development-this privatization of regulatory oversight under the guise of fiscal pragmatism.
When a public health agency begins to derive its operational budget from the very entities it is mandated to regulate, one must ask: Who, precisely, is being served?
Is it the patient? Or is it the shareholder?
The fee structure, while ostensibly tied to cost recovery, has functioned as a de facto barrier to market entry-favoring conglomerates and systematically excluding smaller, innovative firms.
Moreover, the disparity in fees between domestic and foreign facilities, while statistically justifiable, perpetuates a neocolonial dynamic wherein developing nations are penalized for their industrial capacity.
And yet, we are told this is 'transparency'-as if publishing monthly metrics absolves the system of its structural inequities.
Transparency without justice is merely spectacle.
One wonders if, in another decade, we will look back on GDUFA as the moment the FDA surrendered its soul to corporate calculus.
Gayle Jenkins
December 6, 2025 AT 11:57Hey I just want to say I really appreciate how this system is evolving
I know small companies are struggling but the Pre-ANDA program is a real win
My cousin runs a tiny lab in Iowa and they got approved for their first generic last year after two failed attempts
The FDA scientist they met with gave them three specific tweaks to their formulation
They saved six months and $80k
That's not nothing
And yeah the fees are high but if you're a startup you can apply for the discount
It's not perfect but it's the best system we've ever had
And the fact that we're approving 1500 generics a year means millions of people are saving money every day
That's worth fighting for
Darrel Smith
December 7, 2025 AT 01:03They're just making it harder for regular people to get their medicine
Big Pharma doesn't care about patients they care about profit
And now the FDA is their puppet
They charge companies to review their own drugs
That's not regulation that's extortion
And the worst part? The FDA gets richer every year
Meanwhile my dad can't afford his blood pressure pills
So who's really paying the price
Not the companies
Not the FDA
Us
Aishwarya Sivaraj
December 7, 2025 AT 08:51so gdufa is kinda like paying for express lane at toll booth right
you pay extra to get through faster
but what if you dont have money for express lane
you still get there eventually but its slow
and the people who paid are like oh look we got here fast
but what about the rest
also why is india paying more
they make the best generic pills in the world
and now you charge them more for inspecting them
its like charging a chef more because they use better ingredients
confusing
Iives Perl
December 8, 2025 AT 10:02They're using GDUFA to track every pill you take
Did you know the facility fees are tied to RFID tags now?
Every generic pill has a microchip that logs where it was made and who approved it
The FDA is building a national drug database
Next they'll require you to scan your prescriptions before you take them
It's not about speed it's about control
And don't tell me it's not true I've seen the documents
They're already testing it in Ohio
They say it's for safety
I say it's for surveillance
steve stofelano, jr.
December 9, 2025 AT 12:46It is with considerable regard for the public interest that I offer this observation: The Generic Drug User Fee Amendments represent a paradigmatic shift in the governance of pharmaceutical regulation.
By institutionalizing a user-fee model, the Federal Drug Administration has effectively reconstituted its fiscal autonomy, thereby enhancing its capacity to discharge its statutory obligations with greater diligence and precision.
Moreover, the differentiation in facility fees between domestic and foreign entities is not merely economically rational-it is administratively necessary, given the logistical and linguistic exigencies inherent in transnational inspections.
While the concerns of small manufacturers are not without merit, the availability of targeted fee reductions and the Pre-ANDA Program evidences a measured commitment to equity.
Indeed, the transparency measures introduced under GDUFA III constitute a significant advancement in regulatory accountability.
One may reasonably conclude that this framework, while imperfect, serves as a model of pragmatic governance.
Savakrit Singh
December 11, 2025 AT 06:43USA charging more for Indian facilities? 😂
Our factories are better than yours
But you still buy from us because your people are lazy
And now you want us to pay more to inspect our own factories?
Why not just send your inspectors to live here?
Cost? We have 500 inspectors in Hyderabad alone
And you charge $223k? We pay less than $5k to run our QA lab
It's not about cost it's about control
And you call this fairness?
Go cry to your Congress
PS: We make 70% of your pills and you still think we're the problem? 🤦♂️
Cecily Bogsprocket
December 11, 2025 AT 07:41I think what's missing from this conversation is the human side
Behind every generic drug approval is someone who couldn't afford the brand name
Someone who skipped meals to pay for insulin
Someone who waited six months for their blood pressure med
And now, because of GDUFA, that wait is down to three months
That's not just data
That's a person getting better
Yes the fees are high
Yes the system favors big companies
But if we fix those things slowly
At least we're not letting people die while we argue about fairness
Progress isn't perfect
But it's better than stagnation
Jebari Lewis
December 12, 2025 AT 01:23Just want to say thank you to the FDA team for all the hard work
I know you're under pressure
And I know the public doesn't always see how much you do
But I've seen the numbers
Over 2000 foreign inspections since 2012
That's not luck
That's dedication
And the fact that you're still improving the system with Pre-ANDA and Controlled Correspondence?
That's leadership
Yes the fees are high
Yes small companies struggle
But you're trying
And that matters
Keep going