When you pick up a prescription for a generic drug, you expect it to be there. But for millions of Americans, that’s no longer a guarantee. As of April 2025, there are 270 active drug shortages in the U.S.-and the vast majority are generic medications. These aren’t rare glitches. They’re systemic failures that force hospitals to delay cancer treatments, pharmacies to scramble for alternatives, and patients to go without essential medicines. The problem isn’t getting better. It’s getting worse.
Why Generic Drugs Are the Most Affected
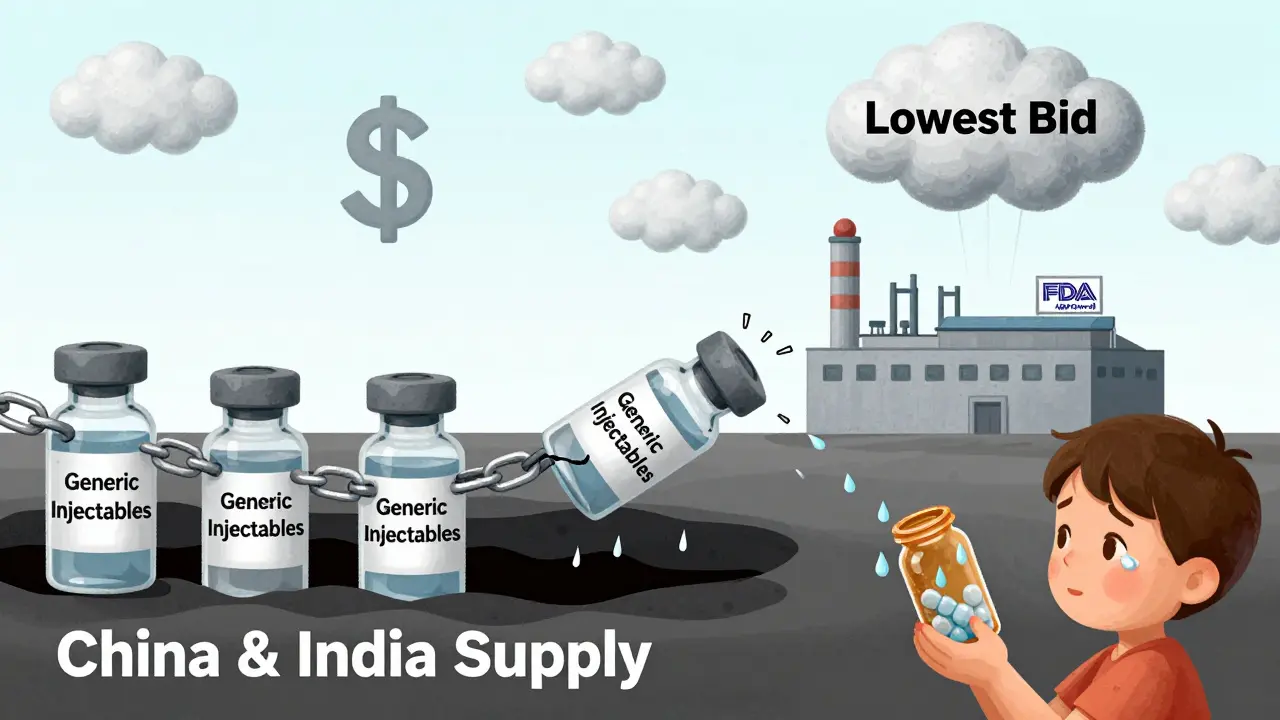
Generic drugs make up about 90% of all prescriptions filled in the U.S. But they account for more than 70% of all shortages. Why? Because the business model for generics is broken. Unlike brand-name drugs, which can charge high prices to recoup R&D costs, generic manufacturers compete on price alone. Profit margins have collapsed. In 2010, the average gross margin for generic drugs was 35%. By 2024, it dropped to 18%. Some sterile injectables-like vancomycin or insulin-earn as little as 5-10% profit. When a factory has a power outage, a contamination issue, or a raw material delay, the company doesn’t have the financial cushion to fix it quickly. They just shut down production and wait for demand to drop.Compounding this, 70% of generic drugs have only one or two FDA-approved manufacturers. If one plant fails, there’s no backup. This is especially dangerous for sterile injectables, which make up 60% of all shortages. These drugs require clean rooms, specialized equipment, and highly trained staff. Building a new facility takes years and millions of dollars. Most companies won’t risk it unless they’re sure they’ll make money-and with prices driven down by competition, they’re not.
The Global Supply Chain Is Fragile
More than half of all drugs used in the U.S. are made overseas. For active pharmaceutical ingredients (APIs)-the core chemical components-80% come from just two countries: China and India. This isn’t just about cost. It’s about control. A single factory in Mumbai or Shanghai can supply hundreds of U.S. hospitals. When the FDA issues a warning letter for poor sanitation or inconsistent quality, production stops. And because these facilities aren’t always inspected frequently, problems go unnoticed until a shortage hits.The median duration of a drug shortage has doubled since 2011-from 12 months to 24 months. That means if you’re missing a drug today, you’re likely to be without it for two years. That’s not a temporary hiccup. It’s a chronic condition. And it’s not just about availability. It’s about safety. When a drug isn’t in stock, doctors turn to alternatives. But those alternatives aren’t always better. Sometimes they’re less effective. Sometimes they cause more side effects. And sometimes, they cost three times as much.
Who Pays the Price?
Patients don’t just lose access to medication-they lose control over their treatment. Oncology departments report having to change chemotherapy regimens because cisplatin or doxorubicin is unavailable. A hospital pharmacist in Texas told a Reddit forum: “We’ve been out of vancomycin powder for eight months. We’re using teicoplanin now. It’s not as reliable, and it costs $1,200 per dose instead of $12.”Chronic pain patients are being denied refills because opioid generics are in short supply. Diabetics are rationing insulin because their usual brand isn’t available. Emergency rooms are seeing more visits because people can’t manage their conditions at home. The National Community Pharmacists Association found that 43% of independent pharmacies have patients abandon prescriptions due to cost or unavailability. That’s not just inconvenient. It’s dangerous.
Healthcare workers are drowning under the weight of managing shortages. Pharmacists spend 15-20 hours a week just finding substitutes, updating electronic records, and training staff on new protocols. Hospitals report that drug shortages make staffing shortages worse-because when nurses and pharmacists are stuck on shortage management, they can’t focus on patient care. The American Hospital Association estimates that U.S. hospitals spend $213 million a year just to cope with these disruptions.
Why Alternatives Don’t Always Work
Brand-name drugs have shortages too, but they’re less disruptive. Why? Because there’s usually a therapeutic alternative. If one brand of blood pressure pill is out, another brand with the same active ingredient is likely available. But with generics, that’s not the case. Many generics are the only version of a drug on the market. When it’s gone, there’s no direct substitute. Doctors have to switch to a different class of drug entirely-like moving from a beta-blocker to a calcium channel blocker for hypertension. That’s not just a change. It’s a gamble.Price spikes are another hidden cost. While brand-name drugs in shortage rarely increase in price (because they’re protected by patents and market control), generic drugs in shortage often jump 14.6% on average. In some cases, the substitute drug costs three times as much. A patient who paid $5 for a generic antibiotic might now pay $15-or $45-if the alternative isn’t covered by insurance. For people on fixed incomes, that’s not an option.
What’s Being Done-and Why It’s Not Enough
The FDA created a Drug Shortage Task Force in 2024 with four goals: diversify manufacturing, create financial incentives, adopt advanced production tech, and improve early warnings. Some progress has been made. The Essential Medicines List, launched in 2020, cut shortages of critical drugs by 32% between 2020 and 2023. But the gains didn’t last. Shortages rose again in 2023 and hit 323 in early 2024 before easing slightly.The real issue? The market still rewards the cheapest supplier, not the most reliable one. Companies that invest in quality, redundancy, and safety are punished by buyers who choose the lowest bid. The FDA itself has seen a 35% increase in manufacturing citations since 2020. That means more plants are failing inspections-not because they’re negligent, but because they’re running on empty. No profit. No reinvestment. No backup.
Proposed tariffs of 50-200% on imported drugs could make things worse. If China and India raise prices to cover new taxes, the cost of APIs will spike. That means even fewer manufacturers will stay in the game. The Congressional Budget Office predicts shortages will hit 350 by the end of 2026 if nothing changes. And most of those will be generic injectables-the kind you need in an ICU, an ER, or a cancer clinic.
The Path Forward
There’s no quick fix. But there are clear steps that could help:- Guarantee minimum profit margins for essential generics, especially sterile injectables, so companies can afford quality control and backups.
- Create a federal stockpile of critical drugs, like the one used for vaccines, to buffer against sudden disruptions.
- Require manufacturers to maintain at least two production sites for high-risk drugs, so one failure doesn’t mean total loss.
- Incentivize domestic manufacturing with tax credits and grants to rebuild U.S. capacity-since the number of FDA-registered generic facilities dropped 22% from 2015 to 2024.
- Change procurement rules so hospitals and insurers don’t always pick the cheapest option. Quality and reliability should count.
Right now, the system is built to save pennies. But it’s costing lives. Every time a patient can’t get their medication, it’s not just a logistical problem. It’s a moral failure. The solution isn’t more reports or more task forces. It’s a shift in priorities-from lowest price to safest supply.
Why are generic drug shortages so common compared to brand-name drugs?
Generic drugs are more vulnerable because they’re sold at very low prices with thin profit margins-sometimes as low as 5-10%. Manufacturers can’t afford to invest in backup production lines, quality improvements, or excess inventory. Brand-name drugs, while also facing shortages, often have higher margins and multiple manufacturers, giving them more flexibility to respond to disruptions.
Which types of generic drugs are most likely to be in short supply?
Sterile injectables are the most affected, accounting for about 60% of all shortages. These include antibiotics like vancomycin, chemotherapy drugs like cisplatin, and IV fluids like saline. They require complex, expensive manufacturing under sterile conditions, and only a few facilities worldwide can produce them. With razor-thin margins, companies avoid investing in redundancy.
How do drug shortages affect patient outcomes?
A 2022 American Medical Association survey found that 63% of pharmacists reported serious adverse patient outcomes due to drug shortages. These include delayed cancer treatments, uncontrolled chronic conditions, increased ER visits for pain or infection, and higher rates of hospitalization. In some cases, patients are forced to use less effective or more toxic alternatives.
Why can’t we just make more generic drugs in the U.S.?
Building new manufacturing facilities is expensive and takes years. The number of FDA-registered generic drug plants in the U.S. dropped 22% between 2015 and 2024. Companies avoid investing because the market rewards the lowest bid, not the most reliable supplier. Without policy changes to guarantee profitability, manufacturers won’t risk the capital.
Are tariffs on imported drugs making shortages worse?
Yes. About 80% of active pharmaceutical ingredients come from China and India. Proposed tariffs of 50-200% would raise the cost of these ingredients, forcing manufacturers to either raise prices (making drugs unaffordable) or stop producing them entirely. Analysts warn this could trigger a wave of new shortages, especially for life-saving injectables.


Jonathan Noe
February 12, 2026 AT 09:08Let me break this down real simple: the generic drug market is a race to the bottom. Companies aren’t evil-they’re just responding to how the system is built. If you force them to compete on price alone, with no profit buffer, of course they’ll cut corners. No one’s going to spend $50 million building a second sterile injectable line when they’re making 8% on each vial. It’s economics 101.
And yeah, 70% of these drugs have only one manufacturer? That’s not an accident. It’s a feature. Why invest in redundancy when the buyer picks the cheapest bid? The FDA can issue all the warnings they want, but until procurement rules change, this isn’t going anywhere.
Also, stop pretending tariffs on China/India are a solution. That’s like burning down your house because the neighbor’s dog barks too loud. It’ll make APIs more expensive, shrink the number of suppliers further, and push even more production overseas-where oversight is even weaker. We’re not fixing the system. We’re just making it more broken.
Jim Johnson
February 14, 2026 AT 02:09Man, this hits hard. I work in a rural pharmacy and we’ve been out of metformin for 11 months now. Old folks are skipping doses, some are switching to insulin because they can’t get the pills. It’s not just about money-it’s about dignity. People shouldn’t have to choose between food and their meds.
Also, the vancomycin thing? Yeah, we’ve been using teicoplanin too. It’s like trying to fix a flat with duct tape. Works sometimes. Not always. And it costs 10x more. Insurance won’t cover it for most patients. So they just go without.
We need a federal stockpile. Like, right now. Not in 2027. Like, now. And stop letting hospitals bid on drugs like they’re buying paperclips. Quality should matter more than price. I’ve seen lives saved because we had a backup. We need more backups.
Vamsi Krishna
February 15, 2026 AT 09:50Bro, this is all India’s fault. Seriously. You think we’re having shortages because of capitalism? Nah. It’s because 80% of APIs come from those two countries-China and India-and they’re just not trustworthy. I’ve seen videos of Indian factories where workers are barefoot, no masks, sewage running next to the vats. That’s not manufacturing. That’s a biohazard.
And don’t even get me started on the FDA. They inspect these places once every 3 years. ONE TIME. Meanwhile, our own U.S. plants are shut down because they can’t compete with dirt-cheap, dirty production. We need to ban all imports. Build everything here. Tax the hell out of foreign manufacturers. Make them pay for the mess they created.
And if you think tariffs are the problem? You’re the problem. We need sanctions. Full blockade. Let them feel the pain. Maybe then they’ll start making quality stuff. Or we’ll make it ourselves. Either way-no more outsourcing life-saving drugs to third-world shitholes.
Brad Ralph
February 16, 2026 AT 03:21christian jon
February 16, 2026 AT 17:03THIS IS A NATIONAL EMERGENCY. WE ARE LETTING PEOPLE DIE BECAUSE WE’RE TOO LAZY TO FIX IT.
Do you know what happens when a diabetic can’t get insulin? They go into DKA. They end up in the ER. They get a $25,000 bill. Then they die. And we sit here talking about ‘profit margins’ like it’s a boardroom meeting at Apple?
And don’t even get me started on the ‘market forces’ crowd. Market forces? Market forces let a single factory in Mumbai supply 300 U.S. hospitals with vancomycin. ONE FACTORY. WHAT IS THIS, THE MIDDLE AGES?
Our government is literally letting people die so we can save $3 on a vial of antibiotics. We have the technology. We have the money. We have the manpower. But we don’t have the COURAGE. And that’s the real crisis.
Every time someone says ‘let the market decide,’ I want to scream. The market decided. And it chose death. Over $3.
Suzette Smith
February 17, 2026 AT 08:59Autumn Frankart
February 18, 2026 AT 02:26They’re not shortages. They’re engineered. Think about it. The FDA, big pharma, and the government? All in cahoots. They want you dependent on expensive brand-name drugs. So they quietly sabotage generics. Why? So you’ll pay more. So they can control you. The ‘manufacturing crisis’? A distraction. The real goal? To make you pay $1,200 for vancomycin instead of $12.
I’ve seen the documents. The FDA has been warned for years. But nothing changes. Why? Because they’re all on the same payroll. Big Pharma owns the FDA. The FDA owns Congress. And Congress owns you.
They’re not failing. They’re succeeding. And if you think tariffs are the problem? You’re being played. This is all part of the plan. Wake up.
Pat Mun
February 18, 2026 AT 13:37Reading this made me cry. Not because I’m emotional-I’m a nurse, I’ve seen a lot-but because this has been happening for years and no one talks about it like it’s real.
I had a patient last month with congestive heart failure. She was on lisinopril. We ran out. Couldn’t find a substitute. She was on the phone with her daughter every day, begging her to find a pharmacy that had it. She cried. I cried. We had to switch her to a different ACE inhibitor, and it gave her a cough so bad she couldn’t sleep. She lost 12 pounds in three weeks.
And the worst part? No one’s mad. No one’s outraged. We just shrug and say, ‘That’s just how it is.’
It’s not just a policy issue. It’s a human issue. Every time we say ‘it’s too expensive to fix,’ we’re saying someone’s life isn’t worth the cost. And that’s not okay. We need to stop thinking of medicine as a product. It’s a right. A basic human right.
I’ve worked in 12 hospitals. I’ve seen this in every single one. We need to act. Not in 5 years. Not in 2026. Now.
Sophia Nelson
February 19, 2026 AT 07:39